Under the CFDA (China Food and Drug Administration) electronic system for supervising and administering production and distribution of all types of pharmaceutical products, each package of a drug product must be identified by a unique electronic supervision and administration code (the “Code“). The Code is composed of a 20-digit number, a bar code and text (such as telephone number and website address).
The Code must be indicated on the exterior of the package itself and identifies basic information such as drug name, manufacturer, approval number, dosage form and strength, date of production, batch number and package size. All drug manufacturers and distributors are required to record the flow path of a drug product from when it is packed until it reaches the end user or medical institution in a centralized national database maintained by CFDA.
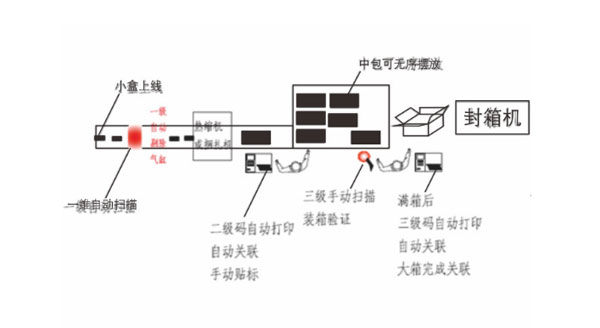
- It adopts the touch screen industrial PC control platform
- It adopts Baiser online image barcode reader (OCR) from Germany, whilst the decoding speed is up to 350 codes/min
- Configured with high-quality online industrial barcode printer
- Satisfy the coding output for hundreds of millions codes
- The ultra-high-speed automatic packaging coding operation
- Configured with the handheld PDA scanners
- Effectively excluding defective products which are lacking labels and/or unreadable
- It adopts the safe and efficient data management mechanism and the authorization management mechanism
- Deployed with the flexible Wan and LAN systems
- It can instantaneously process the data without delay
- Providing with the service to re-develop the interface, which enables the enterprises to reuse the related equipment